Volunteer in a study
The Centre for Integrated Research in Life and Health Sciences (CIRLHS) invites adults of all ages and backgrounds to participate in a range of human research studies. The research conducted in our Centre, under the theme ‘Health, Performance and Physiology’, includes work in the areas of human nutrition, human metabolism (diabetes), human physiology (heat), immunology and the environment.
What to expect
Studies are designed differently depending on the nature of the research. Most studies take place in our laboratories at the University of Roehampton, on our Whitelands Campus (SW15 4JD). Occasionally, studies run online only. The time commitment and number of visits varies. You can find the exact details of what levels of participation are involved under each project description below.
If you change your mind, you are free to withdraw from the study at any time without giving a reason.
Why get involved
Every year, thousands of people in the UK volunteer to take part in research studies. This participation helps to provide valuable knowledge to prevent and treat diseases and help people live healthier lives in the future.
How do I take part?
If you are interested in taking part, please read the inclusion criteria in the individual studies below to ensure you are suitable.
To take part or if you have any questions, you’ll find contact details or website links under each study.
All studies carried out in the CIRLHS are reviewed and approved by the Research Integrity and Ethics Committee.
Title: The role of SlimBiome® (mixture of glucomannan, non-digestible prebiotic fibres and chromium) on glycaemic response to sugary foods
Investigators: Oana Ancu, Drs Richard Mackenzie, Steven Trangmar and Adele Costabile
Summary:
An approach that combines weight loss with additional health benefits is very desirable. SlimBiome® is a functional food ingredient that promotes the feeling of fullness, helps burn calories by promoting a healthy metabolism and maintains blood sugar levels to prevent overeating. It has been shown to reduce calorie intake by up to 20% and lower food cravings. Many diets focus on simply reducing calories in a bid to aid weight loss which is not always sustainable, and commonly leads to failure due to food cravings taking over. In a previous human intervention open-label human study performed at the University of Roehampton, utilizing a meal replacement diet plan over 4 weeks*, SlimBiome® has showed to reduce hunger and therefore takes the will power out of the equation, leading to a more successful and sustainable approach to weight management. This research aims to address that knowledge gap by investigating the effects of SlimBiome® on the glycaemic response to sugary ingredients.
The study will involve five visits to our laboratory at the University of Roehampton (Whiteland College, University of Roehampton). The first visit will last approximately 1 hour, during this time preliminary data on fasting blood glucose and body composition will be measured. The following four visits will each last 3.5 hours in which you will be asked to consume one of the following solutions per trial: 200ml of water, 200ml water/3g SlimBiome or 200 ml water/3g maltodextrin. You will then be asked to drink a glucose drink (50g dextrose in 250 ml of water), approximately 30 minutes later. Blood samples (after 0, 15, 30, 45, 60, 75, 90, 120, 150 min) will be collected as standard practice in our laboratory by finger-prick. Blood glucose levels, changes in cravings and hunger, body composition changes will be monitored during each study visit.
Information for participants:
- You must be male and between 18 and 65 years of age with a Body Mass Index (BMI) 18.5-24.9 kg/m2
- You should have normal blood glucose (4-5.4 mM)
- You must not suffer from any significant health conditions (i.e. neuropathy, nephropathy, retinopathy, vascular diseases, strokes, hypertension, cardiovascular disease), those with anaemia, those who are pregnant, have high blood pressure >140 mmHg systolic/ >90 mmHg diastolic, current smokers, individuals requiring insulin or any other glycaemic altering medication).
On completion of the study, you will receive a £50 Amazon gift card, aiming to compensate for your time in the laboratory and for travelling expenses. Moreover, we will be able to provide you with an accurate measure of your blood glucose levels and body composition.
Title: The impact of seaweeds extract on gut health, immunity and metabolic disorders in healthy adults
Investigators: Enver Keleszade, Drs Michael Patterson, Steven Trangmar and Adele Costabile
Summary:
Studies have shown that significant change to the microbiome due to prolonged stress, medications and diet plays a role in developing metabolic syndrome (MetS). It is estimated that MetS affects 1 in 3 older adults (>50 years) in the UK, and the numbers are climbing. It is a constellation of risk factors comprising abdominal obesity, increased blood pressure (BP), increased fasting plasma glucose (FPG), increased triglycerides (TG), and decreased high density lipoprotein cholesterol (HDL-C) that lead to an increased risk of developing cardiovascular diseases (CVDs), type 2 diabetes mellitus (T2DM) and all cause mortality. Treating MetS is of crucial importance in preventing progression to type 2 diabetes and in reducing mortality and morbidity from type 2 diabetes and CVDs. In recent years, marine organisms, especially seaweeds, have been highlighted as potential natural sources of bioactive compounds and useful metabolites, with many biological and physiological activities to be used in functional foods or in human nutraceuticals for the management of MetS comorbidities. Our first pilot study demonstrated significant reductions in plasma glucose, total cholesterol, LDL cholesterol and increase in HDL cholesterol levels. Along with that, significant reductions were observed in body weight, body fat percentage, fat mass and waist and hip circumferences during the study period of 2 weeks. This study aims to determine the long terms benefits of brown seaweed extracts on gut health, immunity and metabolic disorders.
The study will involve six visits to our laboratory at the University of Roehampton (Whiteland College, University of Roehampton). The first visit will last approximately 1 hour, during this time preliminary data on blood anemia and general medical questions will be measured. The following five visits will each last 40min max in which fasting blood samples will be collected for blood lipids, glucose and liver function analysis. You will be required to consume either the brown seaweed extract or the equivalent placebo controlled capsule three times a day, 30 minutes prior to having a meal, during the treatment period of 12 weeks followed by a 2-week washout period where no products will be consumed.
Information for participants:
- You must not suffer from any significant health conditions (i.e. neuropathy, nephropathy, retinopathy, vascular diseases, strokes, hypertension, cardiovascular disease), those with anaemia, those who are pregnant, have high blood pressure >140 mmHg systolic/ >90 mmHg diastolic, current smokers, individuals requiring insulin or any other glycaemic altering medication).
- You must be females and males, aged 18 years to 65 years with Body Mass Index (BMI) 27 - 35 kg/m2
- Not dieting within the last month and not having lost >5% body weight in the previous year and not increased physical activity levels in the past 2-4 weeks or intending to modify them during the study
On completion of the study, you will receive a £100 Amazon gift card, aiming to compensate for your time in the laboratory and for travelling expenses. Moreover, we will be able to provide you with an accurate measure of your blood chemistry levels and body composition.
Title: Investigating the regulation of Liver Expressed Antimicrobial Peptide 2 (LEAP2)
Investigators: Halimah Rustum, Drs Michael Patterson, Adele Costabile and Astrid Hauge-Evans
Summary:
Obesity prevalence is still increasing across the developed and developing world. Type 2 diabetes is associated with obesity and is one of the largest global health challenges. Hormones produced from the gut, liver and pancreas are important regulators of dietary intake and glucose homeostasis. While most of these hormones are responsible for causing satiety/fullness, there is one peripheral hormone called Ghrelin that stimulates hunger and suppresses insulin release. Therefore, antagonists (blocking) of the ghrelin system may be useful to treat obesity and diabetes. Recently, Liver Expressed Antimicrobial Peptide 2 (LEAP2) was identified as an endogenous(naturally found in your body) antagonist(blocker) of ghrelin. LEAP 2 is thought to be released into blood from the liver and the gut, and early evidence suggests it could have an important role in regulation feeding and glucose homeostasis. We are carrying this study to understand how LEAP 2 is regulated by glucose in healthy individuals and to help understand how regulation may be disrupted in obesity and type 2 diabetes.
Information for participants:
- You must be males and females from 18 to 65 years of age
- Body mass index (BMI) 18 to 25 kg/m2
- You must not suffer from any significant health conditions (i.e. diabetes or from any diseases that may affect their metabolism, digestion, or gut function, not Pregnant or lactating women; not following a weight loss diet)
The study will involve two visits to our laboratory at the University of Roehampton (Whiteland College, University of Roehampton). The first visit will last less than 1 hour, during this time preliminary data on height weight will be taken, and a general medical screening questionnaire will be completed. The following study visit will last 3.5hours maximum. A baseline (0 min) blood sample will be taken then you will be asked to drink a glucose drink (50g dextrose in 250 ml of water). After the glucose drink, blood samples (after 15, 30, 45, 60 and 120min) will be collected using standard practice in our laboratory by venepuncture. Blood glucose levels, changes in hunger and appetite/metabolic hormone changes will be monitored during each study visit.
On completion of the study, you will receive a £20 Amazon gift card, aiming to compensate for your time in the laboratory and for travelling expenses.
For more details please visit the URL https://discoverthesecretofgoodhealth.weebly.com
Title: Effects of hyperthermia on working and long-term working memory function
Investigators: Drs Adam Bruton, Kaz Brandt, Stephen Trangmar, and Chris Tyler
Summary:
Rising global temperatures mean that increasingly individuals are exposed to higher levels of heat stress and, as a result, understanding the consequences of increased heat stress and strain (e.g., body temperature) is of paramount importance. It is well documented that physical performance is impaired in such conditions; however, the relationship between thermal strain and cognitive performance is not well understood. Reductions in cognitive performance (e.g., memory) could markedly impair the ability to work effectively and safety compromising quality of life, safety, and productivity.
This study aims to investigate the effect(s) that passively induced increases in body temperature has on working and long-term memory task performance.
Information for participants:
We are looking for participants who meet the following criteria:
- Male or female
- Healthy
- No known cardiac diseases and/or cardiovascular risk factors
- Non-Smokers
- Do not suffer from Diabetes Mellitus
- Not currently pregnant
- No history of illness, disease or condition that may have significantly affected your brain or your memory
Participants will be required to attend the School of Life and Health Sciences’ Human Physiology Laboratory at the University of Roehampton on one occasion, for no more than 4 hours, at a mutually convenient time.
Participants will be randomly allocated to either the experimental or control group. If allocated to the experimental group, you will be asked to don a water-perfused garment, lie down, and have your core body temperature passively elevated by 1.5°C. If allocated to the control group, you will lie down without the water-perfused suit. Memory performance, body core and skin temperature, heart rate, blood glucose, hydration status, and perceptual data will be recorded during all trials.
For more information, or to take part contact Dr Chris Tyler: Chris.Tyler@roehampton.ac.uk
Title: The role of integrin signalling proteins in muscle glucose uptake and insulin Resistance & the development of type 2 diabetes
Investigators: Diana Motei, Drs Nicholas Hurren, Volker Behrends, Fulvia Draichio and Richard Mackenzie.
Summary:
Type 2 diabetes (T2Ds) is a multifactorial metabolic disease characterized by defects in insulin sensitivity, glucose effectiveness, β-cell function & endogenous glucose production (EGP). It is widely accepted that peripheral insulin resistance precedes β-cell function dysfunction. This research will help to advance our understanding of the defects associated with insulin resistance, a pre-diabetic state.
The study will involve three visits to our laboratory at the University of Roehampton (Whiteland College, University of Roehampton). The first visit will last approximately 1 hour, during this time preliminary data on metabolism and body composition will be measured. The following two visits will be used to perform a saline and insulin infusion (one visit) and a fat and insulin infusion (one visit). The second and third trials will each last circa 7.5 hours (30 minutes preparation, followed by 7 hours of infusion). During these trials, blood and muscle tissue samples will be collected as standard practice in our laboratory.
Information for participants:
- You must not be suffering from any diabetic disease or diabetic related disease conditions (i.e. neuropathy, peripheral vascular and cardiovascular disease), known hypersensitivity to heparins or with previous repeated or regular exposure to heparin
- Physically inactive and active people can participate in the present study, but you must be physically healthy and well, with no communicable blood-borne diseases or clotting disorders.
- You must be male and between 18 and 45 years of age.
During your stay in our facilities, all costs associated with your stay will be covered. Transport costs and lunch will be provided at your request. Moreover, we will be able to provide you with an accurate measure of your metabolism and body composition. For more details please visit the URL www.cfp.cc/2YVIH3
For more further, please contact Alex Rhodes: rhodesa@roehampton.ac.uk
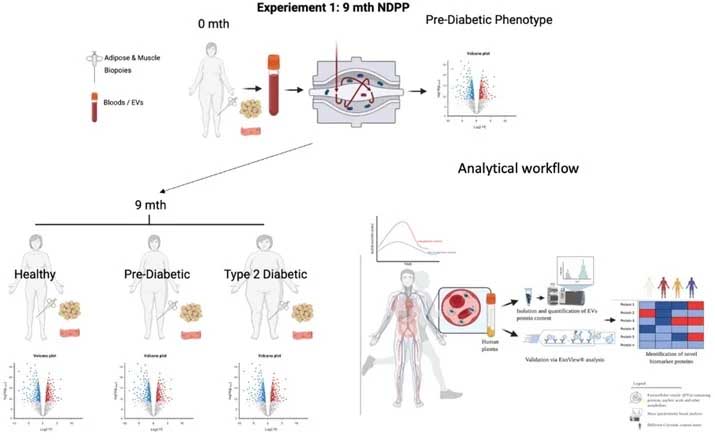
Title: Investigating plasma biomarker molecules associated with the progression of prediabetes to overt type 2 diabetes. An NHS Diabetes Prevention Programme Study
Investigators: Diana Motei, Dr Volker Behrends, Dr Nicholas Hurren and Dr Richard Mackenzie
Summary:
Previous studies have shown changes in the metabolite and protein profiles of pre-diabetic participants in association with the progression of type 2 diabetes but also with the transition to normal glucose regulation (i.e. diabetic remission). Therefore, this study will investigate possible plasma biomarker molecules in pre-diabetic participants for early diagnosis and possibly prevention of type 2 diabetes. We are seeking pre-diabetic individuals to joins this exciting research.
Information for participants:
- You will have a glycated haemoglobin (HbA1c) value between 42 - 47 mmol/mol
- Fasting plasma glucose levels between 6.1 - 6.9 mmol/L
- Blood pressure <140 mmHg systolic/ <90 mmHg diastolic
- You must not be suffering from any complications (i.e. nerve or kidney disorders, damage of the retina, vascular diseases, strokes, persistent high blood pressure, cardiovascular disease, haemophilia), those with anaemia, blood borne diseases, those who are pregnant or in the postpartum period (within 3 months after delivery).
- You must not be a current smokers.
Visit 1 (approximately 2 hours)
You will be provided (verbally) details relating to the study’s purpose, methods and any associated risks. You will be required to read, sign and provide informed consent. This informed consent form will set out full details of the study including risks, safety and benefits. It is important to note that if you agree to volunteer, you are free to leave the study at any point without disadvantage.
Visit 2, 3 and 4 (each visit will last approximately 1.5 hours)
The visits will be carried out at weeks 2, 26 and 36 after the initial visit (visit 1) and will last approximately 1.5 hours. The first hour will be used to deliver the relevant information while the remaining half an hour will be used for in collecting a blood sample and measuring your weight, height and body fat percentage. A blood sample will be collected from a dorsal arm vein by a trained phlebotomist under sterile conditions. A part of the blood sample will be used for measuring HbA1c, glucose and insulin concentration while the remaining plasma will be analyzed for biochemical profiles. Sterile dressings will be applied post blood sampling to reduce the risk of infection. Percentage of body fat will be measured using Bod Pod.
During your stay in our facilities, all costs associated with your stay will be covered. Transport costs and lunch will be provided at your request. Moreover, we will be able to provide you with an accurate measure of your metabolism and body composition. For more further, please contact Diana Motei: moteid@roehampton.ac.uk